Learn about our comprehensive antibody validation methods to ensure monospecificity. Antibody Validation>>
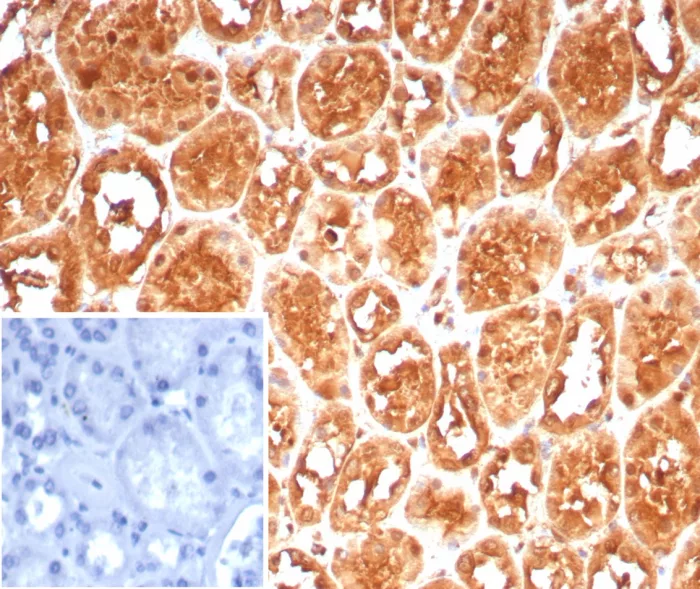
Formalin-fixed, paraffin-embedded human kidney stained with HSPA1A Mouse Monoclonal Antibody (HSPA1A/7932). Inset: PBS instead of primary antibody; secondary only negative control.
This intronless gene encodes a 70kDa heat shock protein which is a member of the heat shock protein 70 family. In conjuction with other heat shock proteins, this protein stabilizes existing proteins against aggregation and mediates the folding of newly translated proteins in the cytosol and in organelles. It is also involved in the ubiquitin-proteasome pathway through interaction with the AU-rich element RNA-binding protein 1.The gene is located in the major histocompatibility complex class III region, in a cluster with two closely related genes which encode similar proteins. [provided by RefSeq]
There are no reviews yet.